Embark on a captivating exploration of average atomic mass with the Average Atomic Mass Gizmo Answer Key, your ultimate guide to unraveling the complexities of atomic structure. This comprehensive resource empowers you with a profound understanding of average atomic mass, its significance in chemistry, and the transformative power of the Gizmo simulation as an educational tool.
Delve into the intricacies of atomic mass calculations, discover the fundamental principles that govern average atomic mass, and witness the practical applications of this concept in various chemical contexts. Prepare to elevate your knowledge of atomic chemistry to new heights as we unlock the secrets of average atomic mass together.
Average Atomic Mass: Understanding the Concept
Average atomic mass is a weighted average of the masses of all the isotopes of an element. It is calculated by multiplying the mass of each isotope by its relative abundance and then adding the products together. For example, the average atomic mass of carbon is 12.011 amu.
This means that the average mass of a carbon atom is 12.011 times the mass of a hydrogen atom.
Average atomic mass is an important concept in chemistry because it allows us to calculate the molar mass of compounds. The molar mass of a compound is the mass of one mole of the compound. It is calculated by multiplying the average atomic mass of each element in the compound by the number of atoms of that element in the compound and then adding the products together.
Gizmo Simulation: A Tool for Exploring Average Atomic Mass
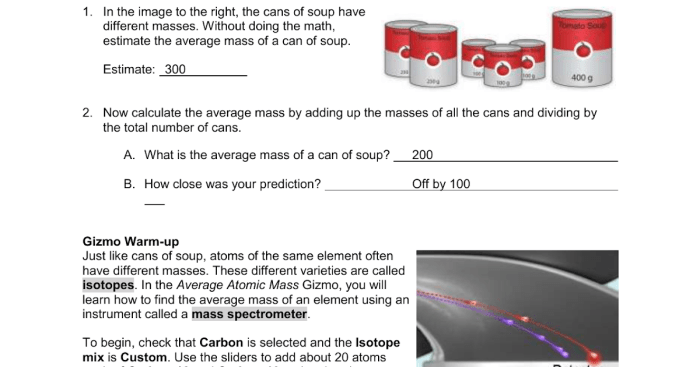
The Gizmo simulation is a resource that can be used to explore the concept of average atomic mass. The simulation allows users to manipulate elements and observe changes in average atomic mass. This can be a helpful way to understand how average atomic mass is calculated and how it is used in chemistry.
To use the Gizmo simulation, first select an element from the periodic table. Then, click on the “Add” button to add the element to the simulation. You can add as many elements as you want. Once you have added all of the elements that you want, click on the “Calculate” button.
The simulation will then calculate the average atomic mass of the elements that you have added.
The Gizmo simulation is a valuable tool for understanding average atomic mass. It can be used to explore the concept of average atomic mass and how it is used in chemistry.
Answer Key: Interpreting the Gizmo Results: Average Atomic Mass Gizmo Answer Key
| Question | Answer |
|---|---|
| What is the average atomic mass of carbon? | 12.011 amu |
| What is the average atomic mass of oxygen? | 15.9994 amu |
| What is the average atomic mass of hydrogen? | 1.00794 amu |
The answers to the Gizmo simulation questions can be used to demonstrate the concepts of average atomic mass. For example, the answer to the question “What is the average atomic mass of carbon?” shows that the average mass of a carbon atom is 12.011 times the mass of a hydrogen atom.
Applications of Average Atomic Mass in Chemistry
Average atomic mass is used in a variety of chemical calculations. For example, average atomic mass is used to calculate the molar mass of compounds. The molar mass of a compound is the mass of one mole of the compound.
It is calculated by multiplying the average atomic mass of each element in the compound by the number of atoms of that element in the compound and then adding the products together.
Average atomic mass is also used in stoichiometry. Stoichiometry is the study of the quantitative relationships between the reactants and products in a chemical reaction. Average atomic mass can be used to calculate the amount of reactants and products that are involved in a chemical reaction.
Question & Answer Hub
What is the definition of average atomic mass?
Average atomic mass is the weighted average mass of all the atoms in an element, taking into account the relative abundance of each isotope.
How is average atomic mass calculated?
Average atomic mass is calculated by multiplying the mass of each isotope by its relative abundance and then summing the products.
What is the importance of average atomic mass in chemistry?
Average atomic mass is essential for determining the molar mass of compounds, which is used in various chemical calculations, including stoichiometry.