Embark on an enlightening journey with the mystery periodic table answer key, a gateway to unlocking the secrets of the elements and their captivating properties. Delve into the periodic table’s intricate organization, unravel the periodic trends, and explore its diverse applications.
This comprehensive guide provides a panoramic view of the mystery periodic table, empowering you with a profound understanding of its elements, properties, and significance.
1. Introduction
The mystery periodic table is a simplified version of the traditional periodic table that conceals the identities of the elements. It is designed to challenge students and foster critical thinking skills by requiring them to deduce the properties and relationships between elements based on their position and clues provided.
The concept of a periodic table organizes chemical elements based on their atomic number, electron configuration, and recurring chemical properties. Elements are arranged in rows (periods) and columns (groups) such that elements with similar properties are grouped together.
2. Elements and Their Properties
The mystery periodic table includes a variety of elements, each with unique properties. These properties can be classified into physical properties (e.g., density, melting point) and chemical properties (e.g., reactivity, oxidation states).
Periodic trends in properties across periods and groups can be observed in the mystery periodic table. For example, elements in the same period generally have increasing atomic number and decreasing atomic radius, while elements in the same group typically have similar electron configurations and chemical properties.
3. Periodic Table Organization
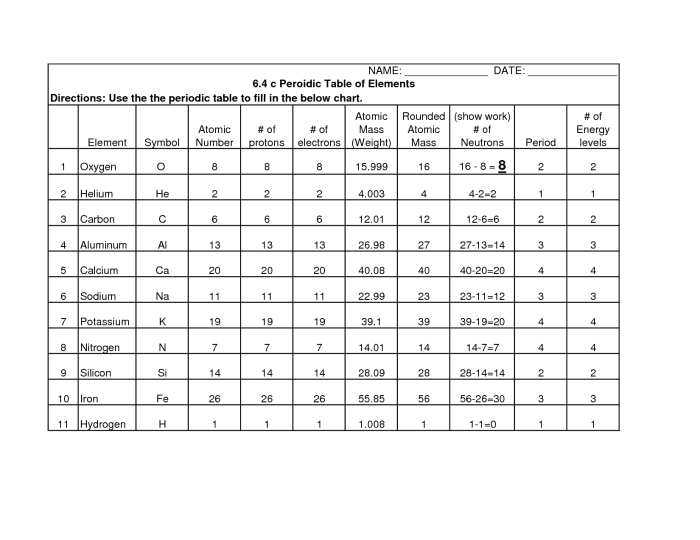
The mystery periodic table is organized based on the atomic number, atomic mass, and electron configuration of the elements. Atomic number, represented by the number of protons in the nucleus, determines the element’s identity and position in the periodic table.
Atomic mass, the weighted average of the masses of an element’s isotopes, is used to calculate the element’s relative atomic mass. Electron configuration, the arrangement of electrons in energy levels around the nucleus, determines an element’s chemical properties.
4. Applications of the Mystery Periodic Table
The mystery periodic table is a valuable tool in chemistry education. It challenges students to apply their knowledge of periodic trends and chemical properties to identify unknown elements.
Beyond education, the mystery periodic table can be used in various fields, including research and industry. It can aid in predicting the properties of new materials, understanding chemical reactions, and developing new technologies.
5. Limitations and Challenges: Mystery Periodic Table Answer Key
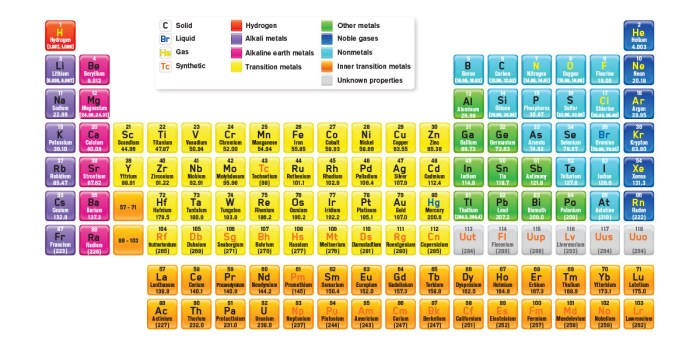
While the mystery periodic table is a useful tool, it has certain limitations. The simplified nature of the table means that it does not provide detailed information about each element.
Additionally, the lack of element names can make it challenging for students to connect the mystery periodic table to the real world. Overcoming these limitations requires further research and development of more comprehensive mystery periodic tables.
FAQ Overview
What is the purpose of the mystery periodic table?
The mystery periodic table is a valuable tool for understanding the properties and relationships between different elements.
How is the mystery periodic table organized?
The mystery periodic table is organized based on atomic number, atomic mass, and electron configuration.
What are some limitations of the mystery periodic table?
One limitation of the mystery periodic table is that it does not include all known elements.